Expert solutions backed by diverse data.
EXPERTISE + INNOVATION
From making initial design decisions through preparing submissions for regulatory bodies, let Design Science’s team structure your research to get you the answers you need.
From Formative…
Discover what users want and how well your product meets their needs to shape your design direction early on.
…to Validation (Summative)
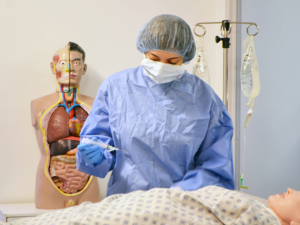
Prepare for submission with a team that not only stays current on necessary standards, but also sits on regulatory committees to ensure that your documents comply with constantly evolving requirements and best practices.
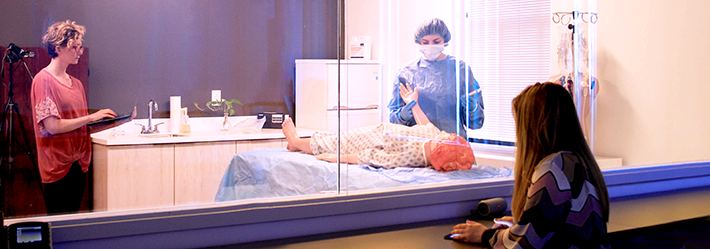
REALISTIC ENVIRONMENTS
State-of-the-art labs, from the home to the hospital.
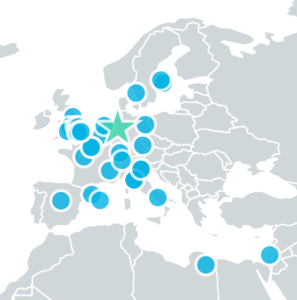
EUROPEAN REQUIREMENTS
When your design isn’t limited to one country, your study shouldn’t be either.
Combined FDA and EMA/CE Mark Approval
Conduct parallel testing—designed to satisfy international requirements—in the US and EU with in-sync study teams. Once the study is over, come away with documents that satisfy both FDA and EMA/CE mark expectations.
THINK OUTSIDE THE BOX
Set Design Requirements
Our customized, proprietary tools help you create design-control documents based on real users’ needs.
Time + Motion
Use comparative, realistic scenarios to reveal advantages or disadvantages that support your marketing claims and product development.
Eye Tracking
Incorporate designers who can update your instructions in real time based on eye tracking data.