MDO Article: The Value Of Eye-Tracking Software In Medical Device Usability Testing
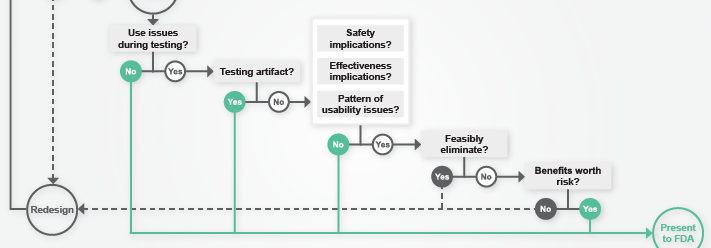
When conducting usability testing, moderators rely heavily upon observations and interviews in order to evaluate medical devices. However, as Data & Systems Analyst Mahajabin Rahman points out in Design Science’s most recent article for MedDeviceOnline, the use of eye-tracking software can support these methods with objective, real-time data. Read more